-
Research & Graduate Education
- About Our Office
- Staff
- Newsletters
- Events
- Sponsored Projects Services
- Research Integrity
- Industry Alliances & Commercialization
- Institutes and Centers
- Graduate Education
- Center for Undergraduate Excellence
- Internal Funding Opportunities
- Policies and Guidance
- Statistical Consulting
- Ask the Experts Virtual Town Hall
- Resources
- Quarterly Research Data
- Archives
» Safety with Animals
In emergency and for safety and security concerns, call Public Safety, (714) 997-6763!
General
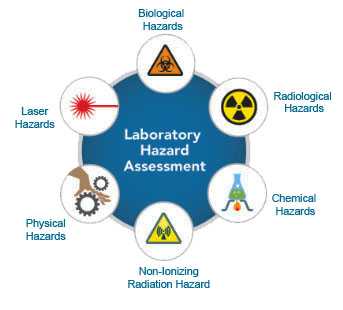
Below is an image that includes a list of hazards (e.g., biological, radiological, chemical, non-ionizing radiation, physical, laser) that should be evaluated during a Laboratory Hazard Assessment.
Toggle Section
In case of an accident...
Notify your supervisor(s), contact Public Safety, and obtain medical services.
Safety & Security Concerns / Public Safety (714) 997-6763
Submit an incident report (on-line, as below) to the University’s Department of Risk Management. Contact the Office of Environmental Health, EHS@chapman.edu.
Submit an incident report online.
Selected Sources of Safety Information and Related Committees
- Environmental Health & Safety (EH&S) office has responsibility over many animal-related functions, including general laboratory safety, biological safety, chemical handling and hazards, radiation and laser safety, emergency management, waste management, respirator training, and occupational health & safety. EH&S helps to access Safety Data Sheets.
- The Institutional Biosafety Committee (IBC), a component of EH&S, is responsible for oversight of studies with concerns about recombinant DNA, select agents, bio-toxins, and other potentially hazardous biological agents. Resources including the Biosafety Manual and Biological Use Authorization (BUA) form are available at the EH&S site. Review by the IACUC of an animal use protocol may require input from the IBC as a condition of approval.
- The Radiation Safety Committee (RSC) reviews and approves applications for Radiation Use Authorization (RUA) to cover use of radioisotopes. The Radiation Safety Officer conducts training on use of radiation-emitting machines. Review by the IACUC of an animal use protocol may required input from the RSC as a condition of approval
- Biosafety in Microbiological and Biomedical Laboratories (BMBL) is produced by the CDC's Office of Health and Safety and the NIH to provide national guidelines to promote the safety and health of workers in biological and medical laboratories.
- Guide for the Care and Use of Laboratory Animals